Like us on Facebook https//wwwfacebookcom/k12mojoFollow us on twitter https//twittercom/K12MojoDownload our App https//playgooglecom/store/appList the points of differences between homogeneous and heterogeneous mixtures Homogeneous Mixture Heterogeneous Mixture Components do not appear separate from one another Components appear to be separate from one another It has a uniform composition It has a nonuniform composition Examples salt solution in water, sugar solution in water Examples MixtureAs a homogeneous mixture has two or more distinct phases Is milk is a homogeneous mixture Why

Write The Difference Between Homogeneous And Heterogeneous Solution With An Example Brainly In
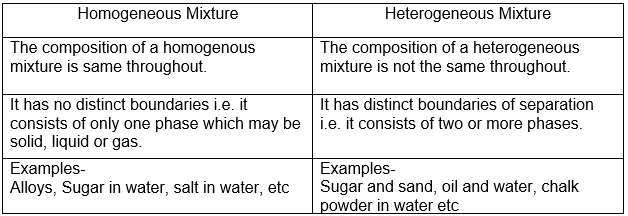
Difference between homogeneous and heterogeneous mixtures with examples class 9
Difference between homogeneous and heterogeneous mixtures with examples class 9-Homogeneous Mixtures Heterogeneous Mixtures centrifugation coagulation distillation evaporation filtration hand picking magnetic separation sieving winnowing sedimentation Mixture Separation Techniques True solution Colloidal solutions Suspensions These solutions typically differ in the particle size of the solute SolutionBased Mixtures True solution solute sizeIn the USA, I learned about the practice of separating students in groups according to their perceived abilities (as judged by educators) It is supposed to help the most able reach their envisioned potential, without being slowed down by those re



1
The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughout The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties Homogeneous and heterogeneous are two different words Here is ur answer Difference between homogeneous and heterogeneous mixture 1) A homogeneous mixture has a uniform composition throughout its mass whereas a heterogeneous mixture has a non uniform composition 2) A homogeneous mixture does not contain physically distinct parts whereas a heterogeneous mixture contains physically distinct
Difference between homogeneous and heterogeneous mixture Class 9 NCERT List the points of differences between homogeneous and heterogeneous mixtures Answer In chemistry, when two or more substances mix with each other without participating in a chemical change, the resulting substance is called a Mixture The difference between heterogeneous and homogeneous mixtures8 Difference Between Homogenous And Heterogeneous Mixture With Examples SHARE Facebook Twitter In physical chemistry and material science, a mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions and colloids One major characteristic of mixtures is that they canAs a homogeneous mixture has two or more distinct phases
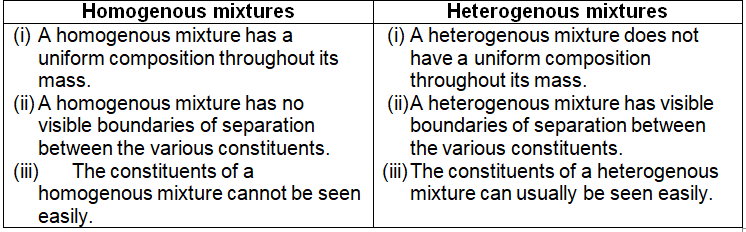
Heterogeneous mixture (I) Homogeneous mixtures have uniform composition throughout the mixture (II) The whole mixture is in same phase (III) Components are not visible to the naked eye (IV) Components cannot be separated easily Eg Sugar Water → Sugar solution (I) Heterogeneous mixture have composition which may vary from point to point What is homogeneous mixture and heterogeneous mixture Class 9?Differences between homogeneous and heterogeneous mixtures Class 9 A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present A mixture is an example of water Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials Stay tuned with BYJU'S to




List The Point Of Differences Between Homogeneous And Hetrogeneous Mixture Brainly In




Classify Each Of The Following As A Homogeneous Or Heterogeneous Mixture Soda Water
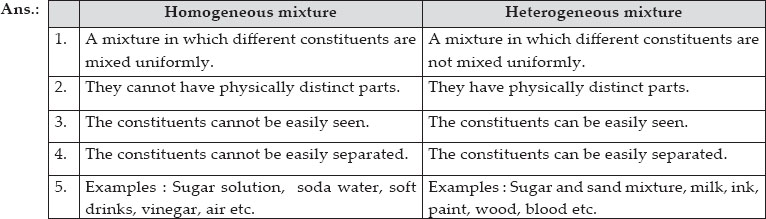
For example, a magnet can be used to separate iron from a mixture of iron and sulphur Mixtures can be homogeneous mixtures or heterogeneous mixtures Homogeneous mixtures Homogeneous mixtures have uniform composition and appearance throughoutA homogeneous mixture is also called a true solution Example sugar solution, ocean water, soft Homogeneous Mixture vs Heterogeneous Mixture A mixture is the combination of two or more pure substance in such a way that they are not chemically united The each pure substance getting into make the mixture have an influence on the mixture as it shows on the type of properties For instance, with making a mixture of sugar and water, the solution made will beHomogeneous Mixture It is a mixture in which different constituents are mixed uniformly and these constituents cannot be easily separated Example Sugar solution, soda, water, soft drinks, vinegar, air, etc But, Heterogeneous mixtures It is a mixture in which different constituents are not mixed uniformly and the constituents can be easily seen and can be easily separated



2




Heterogeneous And Homogeneous Mixture Differences Videos Examples
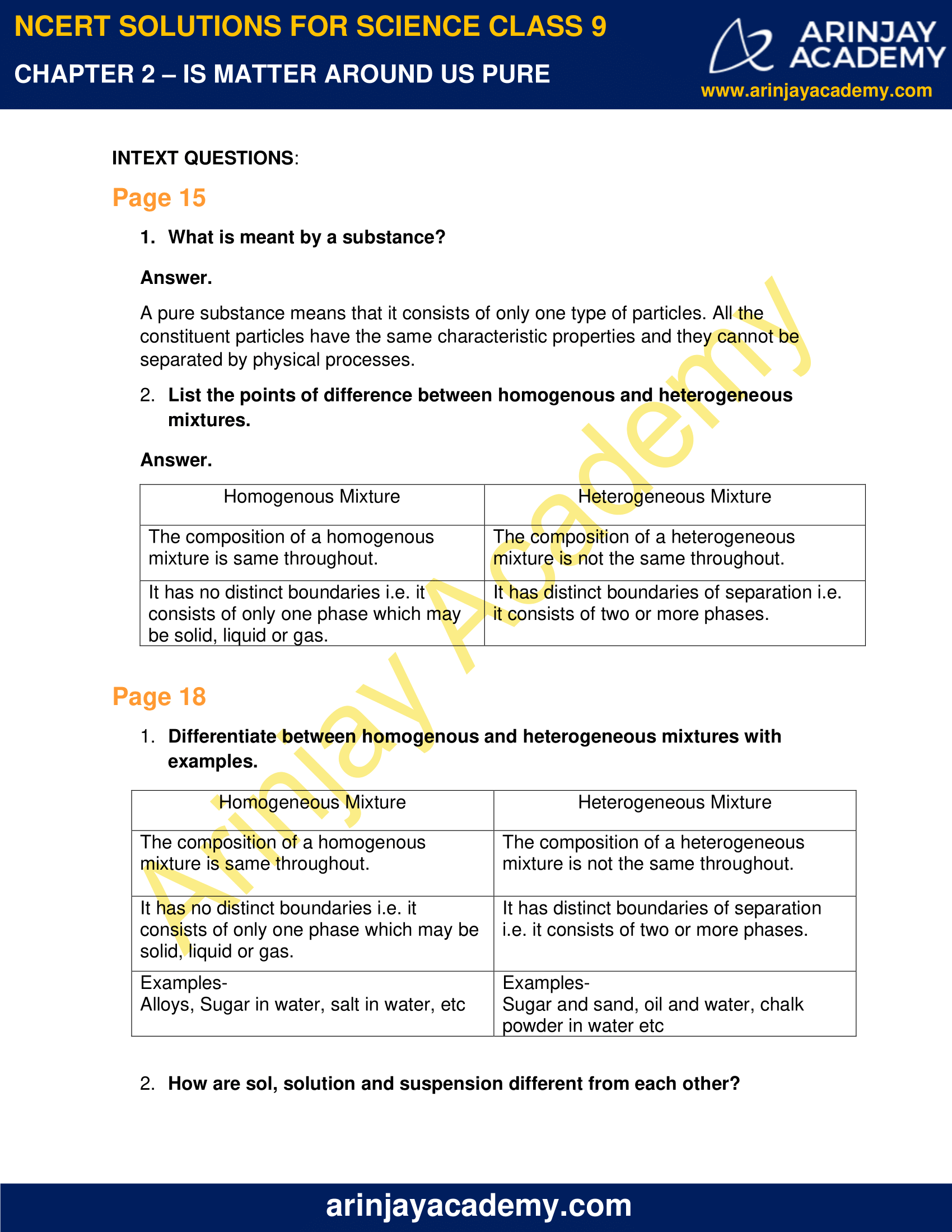
Difference between Homogeneous and Heterogeneous Mixtures ytimgcom Homogeneous and Heterogeneous Mixtures Examples ytimgcom Is Matter Around Us Pure slidesharecdncom Science Class 9 Practice Paper3 with sol for SA 1 dronstudycom Difference Between Heterogeneous & Homogeneous Mixtures tqncom Characteristics of mixtures Mixtures canMixtures which do not have uniform composition throughout are called Heterogeneous Mixture The boundaries of constituent particles of a homogeneous mixture can be identified easily; Question 1 Page 18 Chapter 2 Class 9 Is Matter Around Us Pure (Term 1) Last updated at by Teachoo Differentiate between homogeneous and heterogeneous mixtures with examples




Write The Difference Between Homogeneous And Heterogeneous Solution With An Example Brainly In



2
Difference between homogeneous and heterogeneous mixture 1) A homogeneous mixture has a uniform composition throughout its mass whereas a heterogeneous mixture has a non uniform composition 2) A homogeneous mixture does not contain physically distinct parts whereas a heterogeneous mixture contains physically distinct partsClick here👆to get an answer to your question ️ Differentiate between homogeneous and heterogeneous mixture with examples Join / Login >> Class 9 >> Chemistry >> Is Matter Around Us Pure >> Pure Substances and Mixtures >> Differentiate between homog Question Differentiate between homogeneous and heterogeneous mixture with examples MediumAug 10,21 difference between heterogeneous and homogeneous mixture with examples EduRev Class 9 Question is disucussed on EduRev Study Group by 141 Class 9 Students




Differentiate B W Homogeneous And Heterogeneous Mixtures Teachoo



Ncert Solutions For Class 9 Science Chapter 2 Arinjay Academy
A homogeneous mixture is a mixture where the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughoutExamplesAir,salt,sugar etc On the other hand,a heterogeneous mixture is a mixture where the components of the mixture The difference between a homogeneous mixture and a heterogeneous mixture is that, unlike heterogeneous mixtures, homogeneous mixtures are consistent, meaning their constitution is the same no matter where one looksSoda water, lemonade, a mixture of salt in water are a few examples of homogeneous mixtures Heterogeneous Mixtures – Those mixtures which do not have a uniform composition entirely are heterogeneous mixtures A mixture of oil and water, soil and sand are heterogeneous mixtures as they don't have a uniform composition Difference Between



Http Punainternationalschool Com Assets Upload Ck Images Class ix chemisty july aug material Pdf




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube
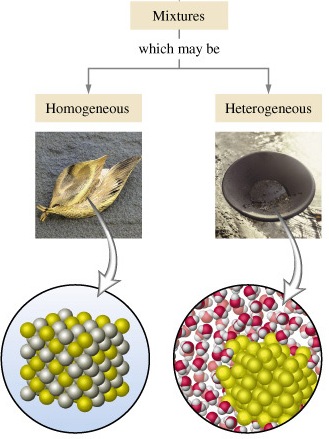
A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present A mixture is an example of water Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials Stay tuned with BYJU'S to learn more interesting topics in ChemistryAn example of a homogeneous mixture is air In physical chemistry and materials science this refers to substances and mixtures which are in a single phase This is in contrast to a substance that is heterogeneous11 A diagram representing at the microscopic level the differences between homogeneous mixtures, heterogeneous mixtures, compounds, and elementsSanjana6169 sanjana6169 Chemistry Primary School Difference between homogeneous and heterogeneous mixture with examples 2




Homogeneous And Heterogeneous Mixtures Card Sorting Activity By Elly Thorsen



Http Sites Isdschools Org Grade6 Remote Learning Resources Useruploads 05 11 Science6 Schimmelsmartwynn May13 Pdf
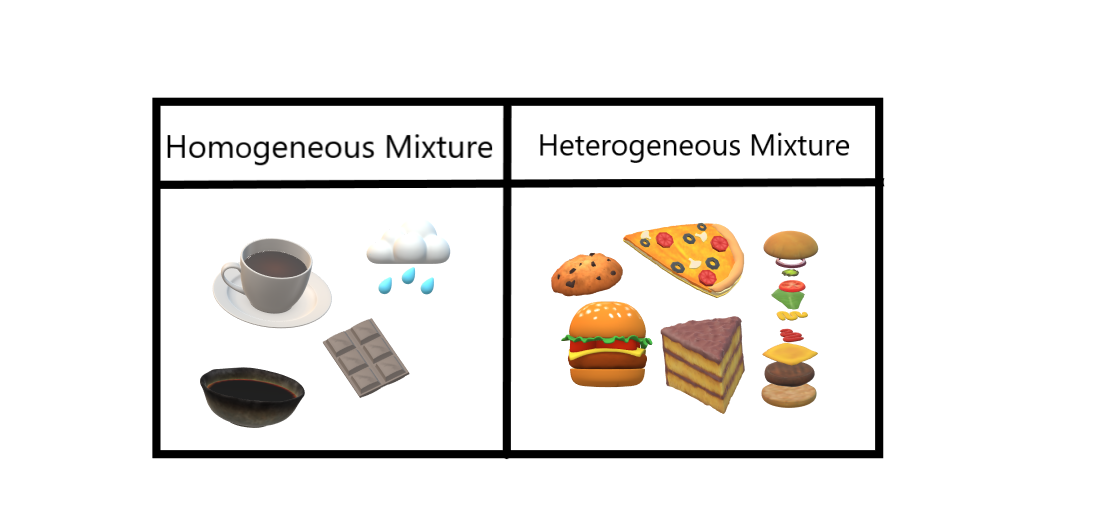
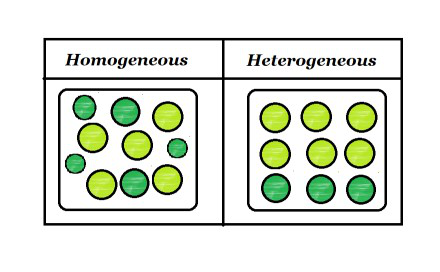
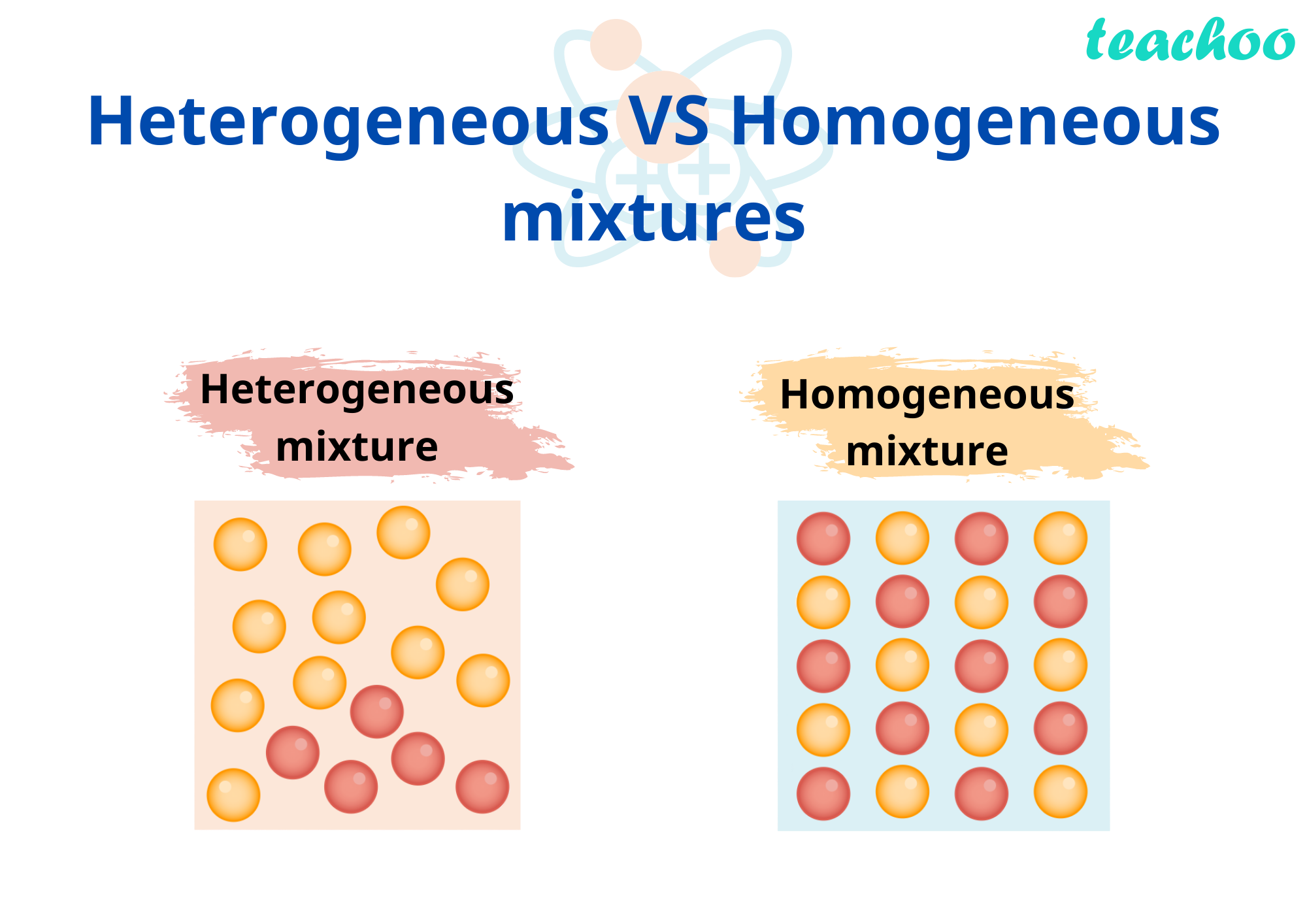
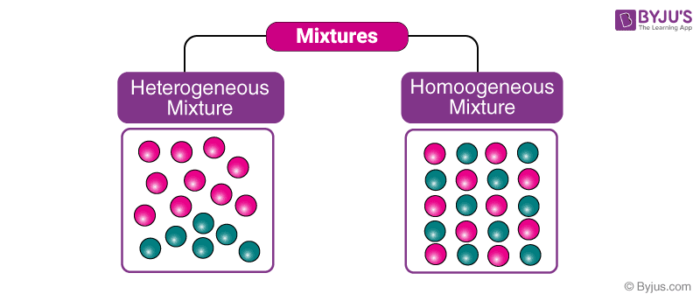
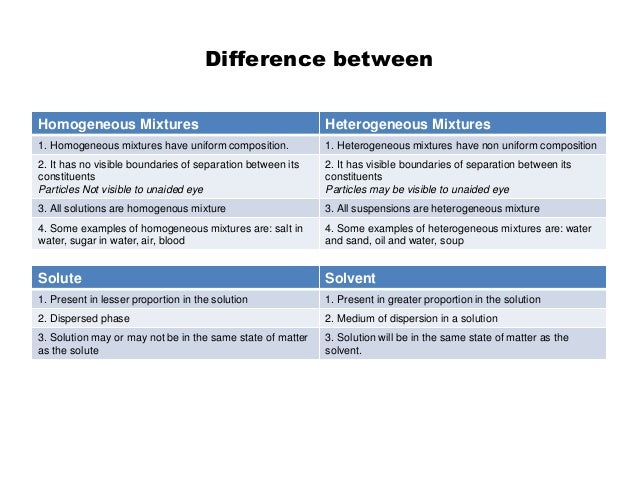
Examples of Homogeneous and Heterogeneous Mixtures Examples of heterogeneous mixtures would be ice cubes (before they melt) in soda, cereal in milk, various toppings on a pizza, toppings in frozen yogurt, a box of assorted nutsEven a mixture of oil and water is heterogeneous because the density of water and oil is different, which prevents uniform distribution in the mixtureSno Homogenous mixture Heterogeneous mixture 1 It has a uniform composition throughout its mass It has no uniform composition 2 It has no visible boundary or boundaries of separation between its constituents For example solution of sugar and salt It has visible boundary or boundaries of separation For example mixture of sugar and sand Difference between Homogeneous and Heterogeneous Mixture Homogeneous mixture Heterogeneous mixture It has a uniform composition It has a nonuniform composition It has only one phase There are two or more phases It can't be separated out physically It can be separated out physically



Ncert Solutions For Class 9 Science Chapter 2 Arinjay Academy




Types Of Mixtures Video Khan Academy
Difference between Homogeneous and Heterogeneous Mixtures 1 Mixtures that have uniform composition Mixtures that do not have uniform composition throughout 2 Boundary of separation could not be seen Boundary of separation of constituent particles is clearly visible 3 Particles are not indistinguishableDifference between homogeneous and heterogeneous mixture with examples Get the answers you need, now!Class9 » Science Is Matter Around Us Pure Dear student, Difference between homogeneous and heterogeneous mixture 1) A homogeneous mixture has a uniform composition throughout its mass whereas a heterogeneous mixture has a nonuniform composition 2) A homogeneous mixture does not contain physically distinct parts whereas a heterogeneous mixture contains




Ncert Solutions Science Grade 9 Chapter 2 Is Matter Around Us Pure
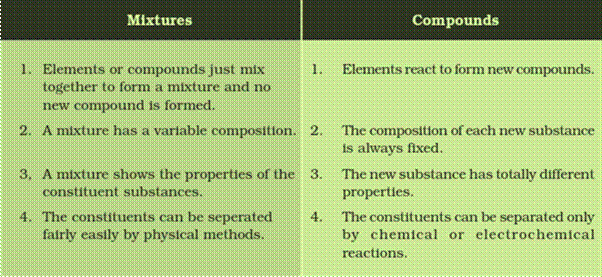



Ncert Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure
The difference between homogeneous and heterogeneous mixture are tabulated below Homogeneous Mixture Heterogeneous mixture They have uniform compositions They have nonuniform compositions The components of homogeneous mixtures are not physically distinct A heterogeneous mixture has physically distinct componentsThe difference between heterogeneous and homogeneous mixtures is the degree to which materials are mixed together and the uniformity of their composition A Homogeneous mixture is a mixture in which components that make up mixture are uniformly distributed throughout the mixture The composition of the mixture is same throughout There is only one phase of matter observed in a homogeneous Homogeneous Mixture vs Heterogeneous Mixture – Types of Solutions An unadulterated substance can be a component, or an intensify that are artificially homogenous in creation and can't be isolated by any physical methods A few instances of an unadulterated substance would be Iron Metal (Fe), Salt (NaCl) and so forth Most characteristic substances, and




List The Point Of Differences Between Homogeneous And Heterogeneous Mixture Brainly In




List The Points Of Differences Between Homogeneous And Heterogeneous Mixtures Write Two Points Of Differences Snapsolve
This video is in simple language about Difference between homogenous and heterogeneous mixturesClass 9Chapter 2Is Matter Around Us Pure A homogeneous mixture is a mixture having a uniform composition throughout the mixture For example salt in water, sugar in water, copper sulphate in water A heterogeneous mixture is a mixture having a nonuniform composition throughout the mixture For example sodium chloride and iron fillings, salt and sulphur, oil and water For moreThe example of salt and water is a classic example because there is no differentiating between the mixture of salt and water The light of passed through the mixture of salt and water is not seen This kind of mixture has a uniform composition that does not separate readily The properties of every part of the homogeneous mixture are the same Below are some homogenous mixture




Food Chemistry Distinguish Between Pure Substances And Mixtures Compare Homogeneous And Heterogeneous Mixtures Define Solutions Distinguish Ppt Download




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com
Differentiate between Homogeneous and Heterogeneous mixture with examples by Jaishree Gorane Leave a Comment List the points of difference between Homogeneous and Heterogeneous mixtures HOMOGENEOUS MIXTURE A homogeneous mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components throughout any given sample HETEROGENOUS MIXTURE A heterogeneous mixture is simply any mixture that is not uniform in composition – it's a nonuniform mixture of smaller constituent partsMixtures which do not have uniform composition throughout are called Heterogeneous Mixture For example – mixture of soil and sand, mixture of sulphur and iron fillings, mixture of oil and water etc The boundaries of constituent particles of a homogeneous mixture can be identified easily;



1
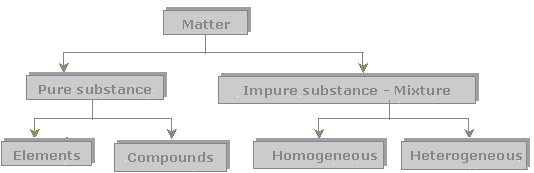



Separating Mixtures Lesson Teachengineering
Example 1 Mixture of Oil and Water is a heterogeneous Mixture We are able to see oil and water clearly separately in the mixture Example 2 Mixture of Salt (Sodium Chloride) and Iron filings is a heterogeneous mixture The particles of salt and Iron filings can be seen and distinguished easily Difference between Homogeneous and Heterogeneous mixtures Homogeneous mixture Heterogeneous mixture 1 Uniform composition Nonuniform composition 2 The nondetectable boundary between solute and solventExamples of homogeneous mixtures include air, saline solution, most alloys, and bitumen A heterogeneous mixture has components in which proportions vary throughout the sample It contains particles of different shapes or sizes and the composition of one sample may differ from that of another sample



Difference Between Homogeneous Mixture And Heterogeneous Mixture



Is Chocolate Chip Cookie Dough Ice Cream Heterogeneous Or Homogeneous
A mixture of alcohol and water is homogeneous while that of oil and water is heterogeneous Explain Explain asked in Class IX By definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




9th Class Chemistry Differentiate Between Homogeneous And Heterogeneous Mixture Brainly In




State The Main Points Of Difference Between Homogeneous And Heterogeneous Mixtures




List The Points Of Difference Between Homogeneous And Heterogeneous Mixtures Brainly In




Homogeneous Mixture Examples In Kitchen




This Presentation Discusses Homogeneous And Heterogeneous Mixtures Provides Examples Explains How A Particle Di Heterogeneous Mixture Pure Products Mixtures



1




Matter Can Be Classified As Mixtures Or Pure Substances Ppt Video Online Download



Homogeneous Mixture And Heterogeneous Mixture Ncert Books




Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Homogeneous Mixture Examples Found At Home



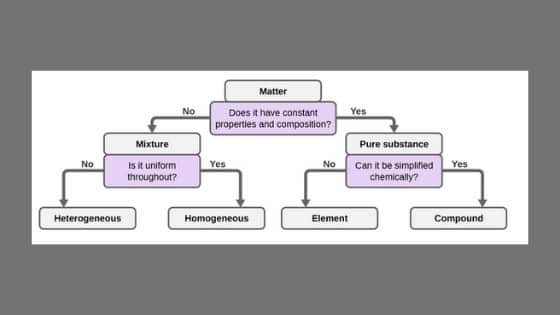
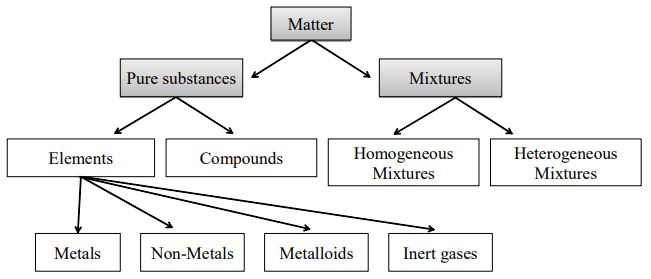
Elements Compounds And Mixtures Class 9 Science Arinjay Academy




Chapter 3 Elements Compounds And Mixtures Frank Modern Certificate Solutions For Class 9 Chemistry Icse Topperlearning




What Is A Homogeneous Mixture Definition And Examples



1




Is Matter Around Us Pure Ncert Solutions For Class 9th Science Chapter 2 Imperial Study




List The Point Of Difference Between Homogenous And Heterogenous Mixtures



What Does It Mean When A Mixture Is Heterogeneous



Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com




Difference Between Homogeneous And Heterogeneous Material Youtube




Write The Differences Between Homogeneous And Heterogeneous Mixtures Brainly In




1 2 The Classification Of Matter The Basics Of General Organic And Biological Chemistry




Is Matter Around Us Pure Solutions




What Is Suspension In Science Definition Types Examples Video Lesson Transcript Study Com




Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture



Http Punainternationalschool Com Assets Upload Ck Images Class ix chemisty july aug material Pdf




Homogeneous Mixture Examples Chemistry




Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous Vs Heterogeneous Youtube




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards



Heterogeneous And Homogeneous Mixture Differences Videos Examples




Which Of The Following Is Not Heterogeneous




Ncert Solutions For Class 9 Science Chapter 2 Is Matter Around Us Pure



Http Www Tiwariacademy Com Wp Content Uploads 15 10 9 Science Ncertsolutions Chapter 2 Intext Page 18 Pdf




Identify The Following As Homogeneous And Heterogeneous Mixtures I Sugar Dissolved In Water Ii Oil And Water
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Distinguish Between Homogeneous And Heterogenous Science Is Matter Around Us Pure Meritnation Com




Examples Of Heterogeneous Mixtures Types Made Simple




Heterogeneous And Homogeneous Mixture Differences Videos Examples




List The Points Of Differences Between Homogeneous And Heterogeneous Mixtures




Differentiate Between Homogeneous And Heterogeneous Mixtures Brainly In




Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Exam Heterogeneous Mixture Chemistry Basics Biology Facts




Differentiate Between Homogeneous And Heterogeneous Mixtures With Examples Youtube




What Is The Difference Between Homogeneous And Heterogeneous Mixture




Q2 Differentiate Between Homog Lido



Is Matter Around Us Pure Practically Study Material




Mixtures Homogeneous And Heterogeneous Mixtures Ppt Video Online Download



Homogeneous And Heterogeneous Mixture Nine Science



What Are Some Examples Of Heterogeneous And Homogeneous Mixtures




Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Youtube



Is Sugar A Homogeneous Or Heterogeneous Mixture Chemistry Point




What Is A Heterogeneous Mixture Definition And Examples




10 Examples Of Mixtures




Homogeneous Mixture Examples In Daily Life




Chemistry For Kids Chemical Mixtures



1




Difference Between Homogenous And Heterogeneous Mixtures Brainly In
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Write 5 Difference Between Homogeneous And Heterogeneous Science Is Matter Around Us Pure Meritnation Com




Difference Between Homogeneous And Heterogeneous Equilibrium Compare The Difference Between Similar Terms




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




What Is The Difference Between Homogeneous And Heterogeneous Mixtures




List The Point Of Difference Between Homogeneous And Heterogeneous Mixture Science Is Matter Around Us Pure Meritnation Com



Is Matter Around Us Pure




1 Differentiate Between Homogon Eous Aid Heterogeneous Mixt Scholr




What Is The Difference Between Material And Substance Quora




Difference Between Homogenous And Heterogenous Mixture



Examples Of Solution Mixtures




Homogeneous Or Heterogeneous Mixtures Practice Worksheet



Cbse Class 9 Science Is Matter Around Us Pure Notes Set B Concepts For Science Revision Notes




Classify The Following As Pure Substances Or Mixtures Separate The Pure Substances Into Elements Compounds And Divide The Mixtures Into Homogeneous And Heterogeneous I Air Ii Milk Iii Graphite Iv Gasoline




Difference Between Homogenous And Heterogenous Mixture




What Are Homogeneous And Heterogeneous Mixtures Give One Example For Each




Write The Difference Between H And Heterogeneous Mixture Brainly In




List 5 Difference Between Homogenous Anf Heterogenous Mixture Brainly In




What Is Difference Between Heterogeneous And Homogeneous Brainly In




Classify Mixtures As Homogeneous Or Heterogeneous Worksheet



0 件のコメント:
コメントを投稿